Effective engineering of a ketoreductase for the biocatalytic synthesis of an ipatasertib precursor
In Communications Chemistry we report the evolution of a ketoreductase that catalyzes a key synthesis step - the reduction of the prochiral ketone to the desired (R,R)-trans alcohol intermediate - with high efficiency in an industrial set-up.
Ipatasertib is a potent, highly selective, small-molecule inhibitor of protein kinase B (Akt) under development by Genentech/Roche for the treatment of certain forms of prostate and breast cancer. The molecule contains three stereocenters and is synthesized in ten synthesis steps using highly selective metal and enzyme catalysts. In a collaboration between F. Hoffmann-La Roche Ltd and the ZHAW Biocatalysis Group a ketoreductase was evolved that catalyzes a key step within the synthesis - the reduction of the prochiral ketone to the desired (R,R)-trans alcohol intermediate - with high efficiency at industrial scale.
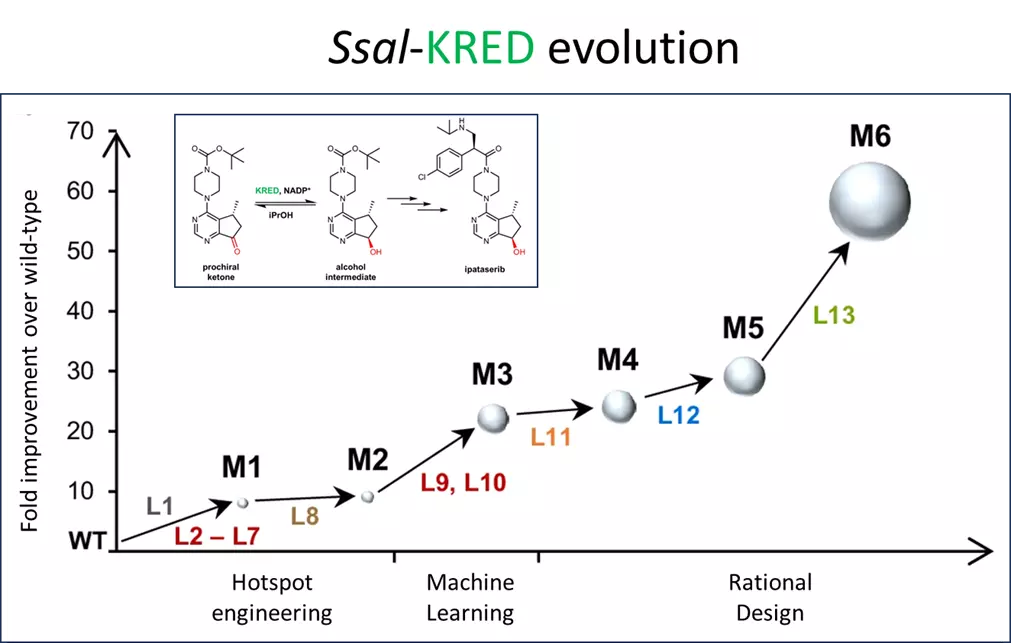
The optimization process started by identifying a suitable enzyme scaffold from a KRED toolbox available at the Laboratory for Biocatalysis (ZHAW). Ketoreductases (KREDs) can stereo-and regioselectively transfer a hybride from NAD(P)H to a carbonyl group of the substrate molecule. A NADP+-dependent aldehyde reductase II originating from Sporidiobolus salmonicolor (Ssal-KRED), described in the literature for processing various alkyl or aryl ketones was chosen as an initial scaffold, as it showed promising conversion rates and exquisite diastereoselectivity (99% de (trans)).
The enzyme was then improved within six rounds of evolution. In total 13 libraries were built as outlined in the figure below). The initial focus was on engineering hotspots that are involved in substrate binding and/or forming the substrate entrance tunnel (HSE, L1-L7) resulting in Mutant M1, which was further improved by changes on the protein surface. Data from the hotspot engineering approach were used to build an algorithm for machine learning based predictions. The number of proposed amino acid changes was reduced by fitting taking into account knowledge obtained from the previous engineering attempts. This way the screening burden was reduced remarkably. The best variant obtained (M3) was further improved by three rounds of iterative combinatorial site mutagenesis (CSM) for performance under industrial process conditions. The final variant (M6) obtained after six rounds of evolution had ten amino acid substitutions and a 64-fold improved activity (Kcat) compared to the starting variant. It showed a conversion rate of ≥ 98% with 99.7% (R,R-trans) diastereomeric excess at 100g/L substrate.
The article published in Communications Chemistry shows that an engineering strategy combining enzyme hot spot identification, mutational scanning libraries and structure guided libraries that can be used to train machine learning algorithms allows to develop effectively a high-performance, industrial biocatalyst.
Reference:
Effective engineering of a ketoreductase for the biocatalytic synthesis of an ipatasertib precursor
Malca SH, Duss N, Meierhofer J, Patsch D, Niklaus M, Reiter S, Hanlon SP, Wetzl D, Iding H & Buller R (2024) Communications Chemistry 7, 46