SELFCAT: Understanding self-regeneration of perovskite-type oxides to prepare active and stable catalysts
At a glance
- Project leader : Davide Ferri, Dr. Andre Heel
- Project status : completed
- Funding partner : SNSF
- Project partner : Davide Ferri
- Contact person : Andre Heel
Description
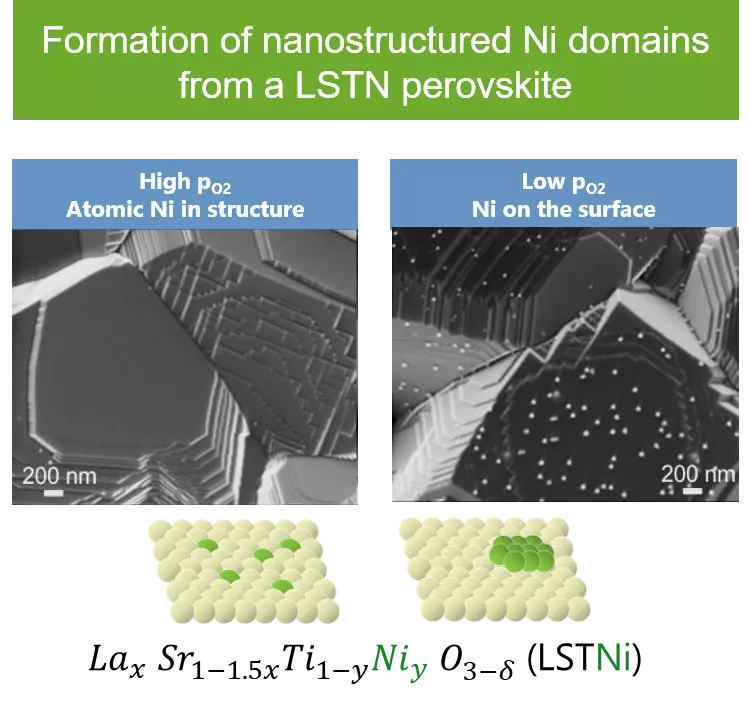
Catalyst deactivation is a key issue in catalyst development. Numerous catalytic processes are limited by deactivation due for example to growth and/or loss of the active metal phase as well as poisoning due to carbon formation or deposition of elements, e.g. sulfur or phosphorous. The principal aim of the project is to understand and apply the self-regenerating function of perovskite-type oxides in order to counteract catalyst deactivation due to microstructural changes of the active phase particles and simultaneous carbon deposition (coking).Perovskite-type oxides are able to segregate metal atoms out of their crystal structure under reducing conditions and to reversibly introduce them into the structure under oxidizing conditions. The major implication of this function is the preservation of the metal particle size from particle growth upon exposure to high operation temperature and reducing environment that is encountered in various catalytic processes. The utility of this function has not been demonstrated for applications other than environmental catalysis. In this project, we aim at demonstrating that this function can be exploited in a similar way to produce active and stable catalysts based on Ni, which is common in a number of applications. The approach is based on the synthesis of a perovskite-type phase in which the active metal only partially substitutes the B-site cation, is homogeneously dispersed and adopts the octahedral coordination environment. Under pre-reduction, or alternately under the reducing conditions imposed by net reducing reaction conditions, the active metal is forced to segregate and to form metal nanoparticles. After oxidation at the same or higher temperature, the active metal phase is protected by re-incorporation into the perovskite-type structure until the next reduction step is applied. CO2 hydrogenation is selected as the probe reaction, since it is a catalytic process under net reducing conditions and moderate but elevated temperatures.