Just published in ACS Catalysis: Modulating Chemoselectivity in a Fe(II)/α-Ketoglutarate-Dependent Dioxygenase for the Oxidative Modification of a Nonproteinogenic Amino Acid
Fe(II)/ α-KGDs are valuable tools for region- and stereoselective derivatization of non-native substrates to pharmacological relevant small molecules.
The article “Modulating Chemoselectivity in a Fe(II)/α-Ketoglutarate-Dependent Dioxygenase for the Oxidative Modification of a Nonproteinogenic Amino Acid“ by Fabian Meyer, Raphael Frey and Rebecca Buller (Competence Center for Biocatalysis, ZHAW) and Mathieu Ligibel, Emine Sager, Kirsten Schroer, Radka Snajdrova (Novartis Institute for BioMedical Research, Basel) is accepted for publication in ACS Catalysis.
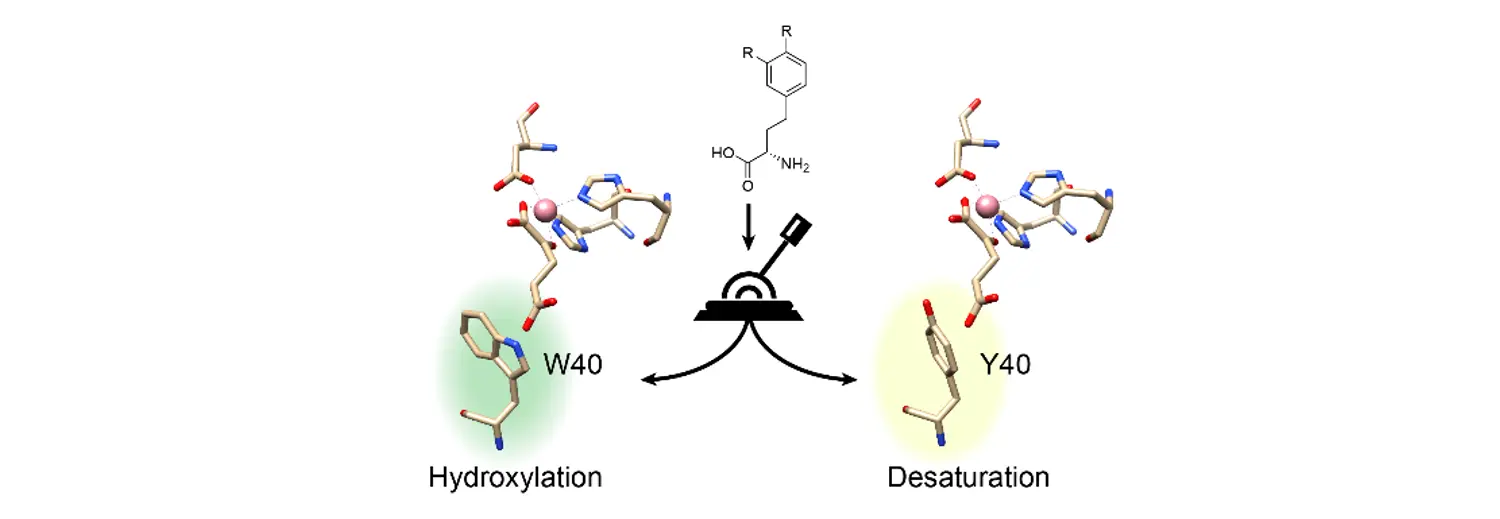
The stereo- and regioselective modification of aliphatic C-H bonds is a challenge for traditional synthetic chemistry. Nonheme iron and α-ketoglutarate-dependent dioxygenases (α-KGDs) are enzymes able to catalyze mild and selective C-H functionalization. The authors screened a library of wild-type Fe(II)/ α-KG dependent dioxygenases and found a variant with hydroxylase activity towards L-homophenylalanine (L-hPhe). A structure-guided semirational protein engineering approach resulted in enzyme variants with significantly improved hydroxylation activity. A single amino acid (W40) turned out to be important for the chemistry taking place - hydroxylation (W40M) or desaturation (W40Y).
γ-hydroxylated L-hPhe is a versatile precursor molecule. Under acid conditions the lactone product forms. An enzymatic cascades leads to two additional side-products – a γ-ketone and a β-hydroxy-γ-ketone. A double mutant (W40M I103L) derived from a second round of engineering showed improved activity compared to W40M and increased conversion to β-hydroxy-γ-ketone.
Furthermore, the authors showed that the new enzyme variants would in addition transform other non-native substrates e.g., L-homotyrosine or (S)-α-amino-3,4-dichlorobenzenebutanoic acid to pharmacological relevant small molecules.