SERAN – Self-Regenerating Anodes: Durability Improvement of SOFC Technology by Novel Smart Materials with Sulphur Tolerance
Beschreibung
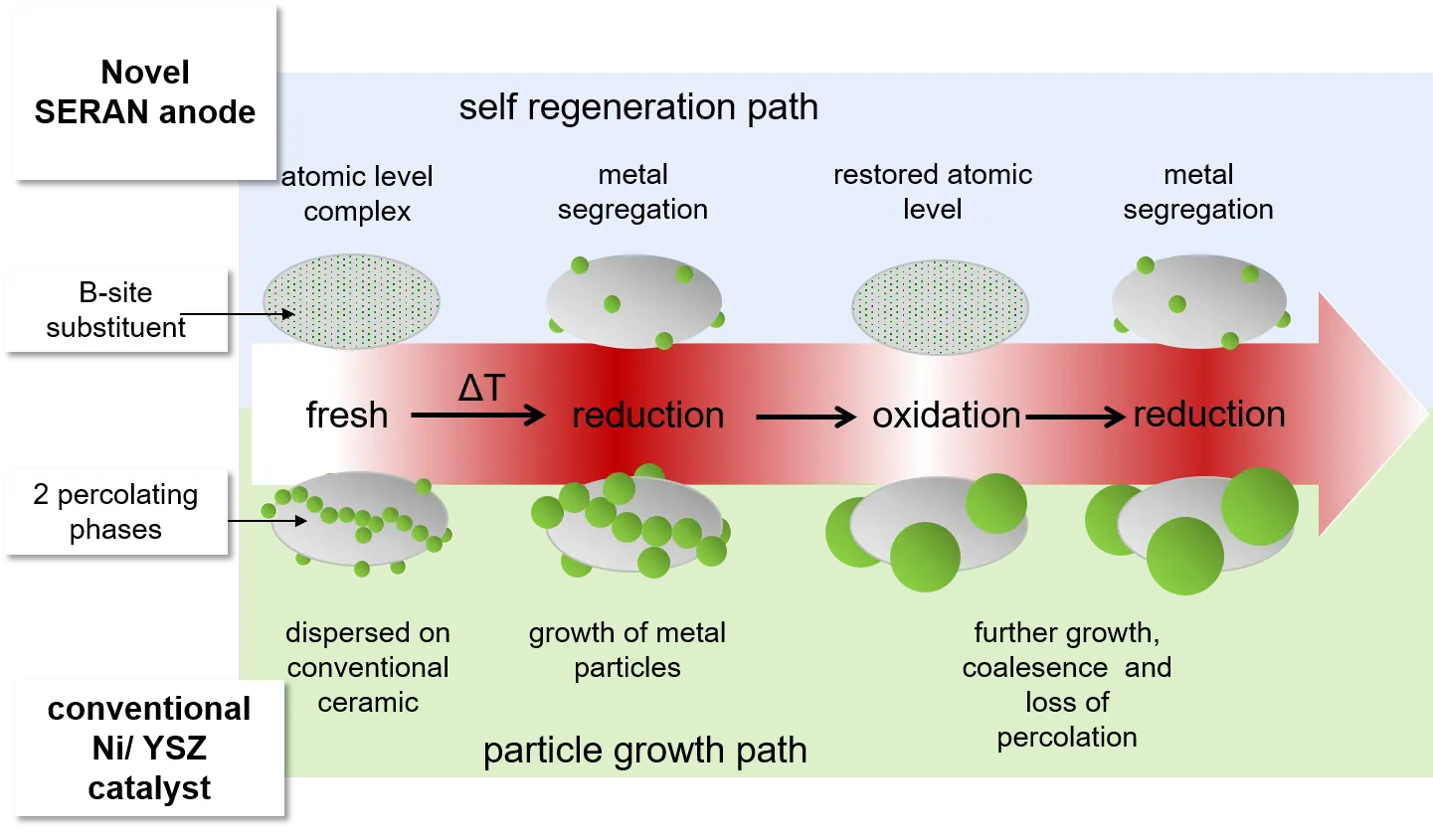
The overall aim of this consortium is the development and demonstration of a novel sulphur tolerant catalyst, which will be applied as catalytic active anode material in SOFCs. The here aimed new anode catalyst has to withstand common sulphur contamination levels of about 30 mg/m3 in natural gas fuels, while state-of-the-art nickel catalysts in Ni/YSZ anodes suffer from instantaneous and additionally irreversible degradation by sulphur traces. Higher sulphur levels can be tested with respect to higher contaminations in renewable biogas fuels. The catalysts are examined with respect to its H2 and CO conversion performance under SOFC anode operation conditions.Initially, main emphasize is set on the development, evaluation and testing of a new ceramic catalyst on the basis of B-site substitution of titanium with nickel in lanthanum doped strontium titanates (LST). Introduction of a second well-defined catalytic active metallic species at the B-site will provide intrinsic sulphur tolerance to the nickel. By this strategy the catalytic active nickel amount willl be reduced by approximately 80% but without minimising the catalytic performance. This ambitious aim allows to introduce a novel self-regenerative effect, but will also reduce cell rupture caused by mechanical stresses during nickel oxide formation. However, still a high electronic conductivity of about 150 S/cm has to be realized for the LST, a factor of 2 - 4 times higher than current ceramic materials. By the use of titanate based perovskites an innovative, self-regenerating effect can be used to limit microstructural and catalytic degradation: redox cycling. Redox cycling – commonly causing an unavoidable degradation of Ni/YSZ and finally a total failure of the cell – is actively used now: it stimulates at first the incorporation of the degraded metallic phase into the perovskite accompanied by a total oxidation of sulphur species to SO2, followed by segregation of a regenerated, because nanostructured and highly dispersed, fresh catalytic active phase. While state-of-the-art Ni/YSZ can withstand ~10 – 13 redox cycles, it is here aimed for 30 and more redox cycles without substantial microstructural and catalytic degradation with respect to ASR and/or cell voltage. A non-progressive degradation by sulphur poisoning of less than 5%/500h is aimed which can be recovered by redox cycling.Finally, on a cell level the complementary use of a mixed ionic electronic conductor (MIEC) together with LST will promote an electrochemical oxidation of sulphur species to SO2. The use of perovskite-type LST and the modified B-site Me phase combine all required strategies to address the following issues: 1) provide sulphur tolerance,2) limit catalytic degradation and self-regeneration of reactive sites which are blocked for the water gas shift reaction,3) avoid microstructure degradation by sulphur and redox cycling,4) avoid the use of costly and service intensive desulphurization cartridges, which are presently a must for the used state-of-the-art-materials.
Eckdaten
Projektleitung
Dr. Andre Heel
Stellv. Projektleitung
Dr. Andreas Mai
Co-Projektleitung
Dr. Dariusz Artur Burnat, Dr. Davide Ferri, Dr. Lorenz Holzer
Projektteam
Hossein Madi, Steiger Patrick
Projektpartner
Paul Scherrer Institut PSI; Ecole polytechnique fédérale de Lausanne EPFL; Hexis AG
Projektstatus
abgeschlossen, 04/2014 - 12/2019
Institut/Zentrum
Institute of Materials and Process Engineering (IMPE); Institute of Computational Physics (ICP)
Drittmittelgeber
Competence Center for Energy and Mobility CCEM-CH; Dritte
Publikationen
-
LST27 anodes for SOFCs : redox stable and sulfur tolerant material
2018 Madi, Hossein; Burnat, Dariusz Artur; Mai, Andreas; Heel, Andre; Van Herle, Jan
-
SMART catalyst based on doped Sr-titanite for advanced SOFC anodes
2016 Burnat, Dariusz; Kontic, Roman; Holzer, Lorenz; Schuler, Andreas; Mai, Andreas; Heel, Andre