Areas of expertise Cell Physiology and Cellular Engineering
Our application-oriented research topics focus on the areas of stem cell research, cell-based test systems, cell differentiation, glycobiology and molecular cell biology (genetic engineering).
Existing scientific competence of the group Cell Physiology & Cellular Engineering are:
Development of cell-based test assays
We actively collaborate with project partners to develop customized standard cell-based assays or improve already existing ones. Examples of assay developed during previous collaborative projects are listed below.
Caco-2 permeability assay
Caco-2 cells (human colon adenocarcinoma) are used to mimic intestinal endothelium cells with their transport and resorption characteristics. The permeability assay performed with this cell line is a standard method used to test drugs and other substances for their resorption capabilities in vitro. Culturing the cells over a period of three weeks on permeable membranes leads to polarized cells expressing a range of different transport proteins on its cell membrane. One of the most prominent is P-gp (P-glycoprotein) which is able to pump out many foreign substances and can therefore be responsible for the development of resistance to drug treatments.
A permeable membrane is separating the culture vessel into two compartments. Therefore two directions of transport can be distinguished. By applying the test compound to one compartment the transport can be determined if the compound concentration is measured after a certain time in the other compartment. The measurements from the apical-to-basolateral (A → B) and basolateral-to-apical (B → A) transport assays allow the determination of the net flux.
Cytotoxicity assay
Medical products, drugs and chemicals are often being tested for their cytotoxicity to determine the risk they might have on humans and on the environment. The bio-compatibility of substances is of special interest for the group of cell physiology and cellular engineering. We perform a cytotoxicity standard assay routinely in vitro on a broad range of different cell lines.
The assessments of the in vitro cytotoxicity assays on cell cultures are based on the preparation of the tested materials. The preparation is then applied to the standardized cytotoxicity assay. The quantification of cell growth is performed by a colorimetric assay (MTT). A toxic effect of the applied substances is assessed by the fitting of a dose-response curve to the growth of the cells to the concentration of applied substance. Furthermore the inhibitory effect can be calculated by the IC50-value, the inhibitory concentration where the growth of cells is reduced to 50% of the control. The results are then finally summarized in a report.
Osteoclast differentiation
Osteoclasts and osteoblasts are responsible for the remodelling of bone by breaking down and rebuilding bone material continuously. A tight regulation has to be established to keep the balance. Cancer metastasis can lead to imbalances by stimulating the increased formation of osteoclasts often leading to osteoporosis within cancer patients. At the lab of cell biology we have developed a test system monitoring the differentiation of osteoclasts by using the mouse macrophage cell line RAW 264.7. With our test system we can determine the differentiation rate of osteoclast quantitatively using a flow cytometer. This method allows us to test more precisely the inhibitory effects of drugs on the differentiation of macrophages to osteoclasts.
Generation of novel antibodies towards complex proteins
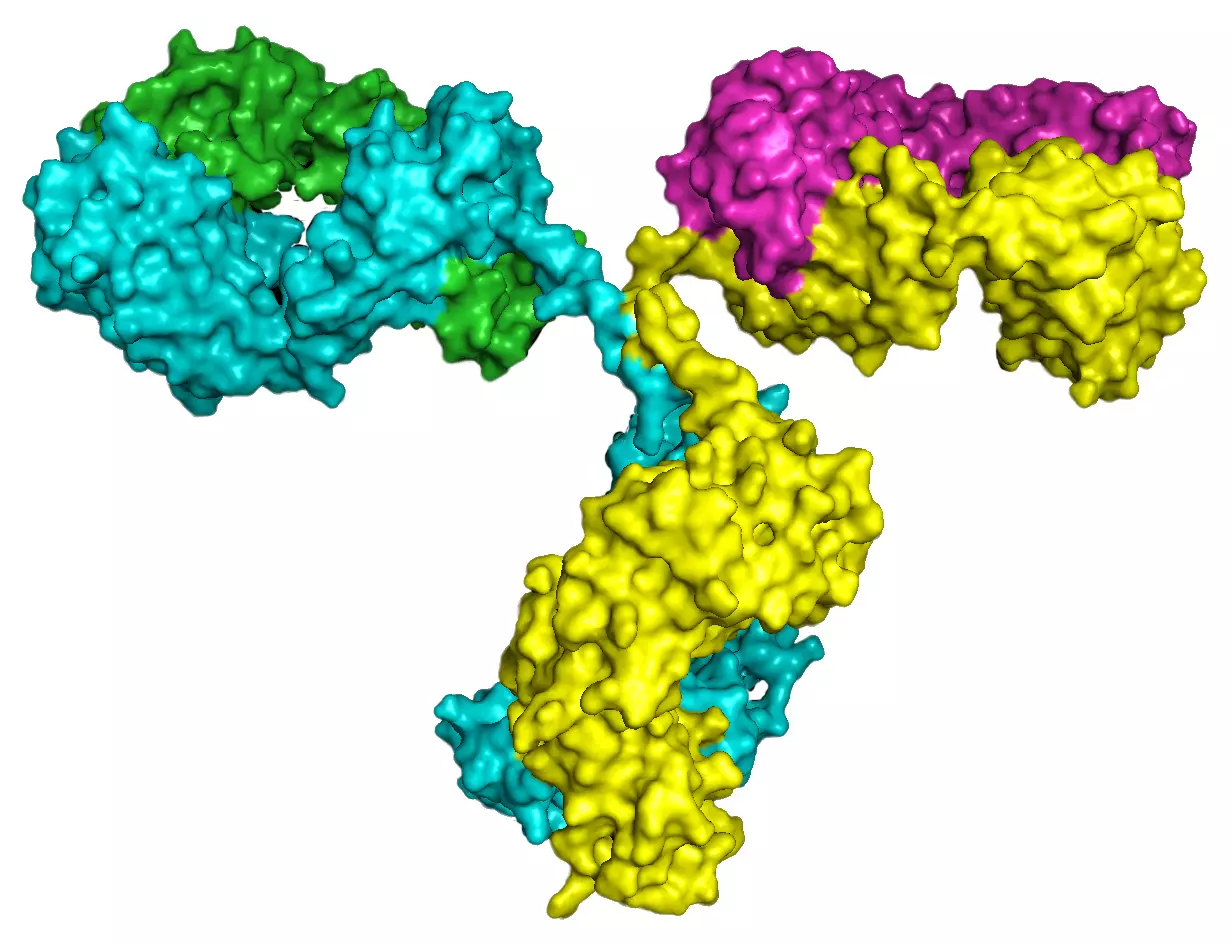
Many clinically relevant proteins such as G-protein coupled receptors (GPCRs) or ion channels have a complex structure with multiple membrane spanning regions. Therefore purification of the proteins as a source of antigen to raise specific antibodies is not feasible. Peptides representing a very small fraction of the protein of interest are not in the native conformation and often lead to lower affinity antibodies. Our method relies on the fact that the full-length human protein in its native conformation is used as an antigen which results in the generation of high affinity antibodies. Additionally, we use rabbits for the immunization since it is known that rabbits produce in general higher affinity antibodies than mice which also helps to generate excellent antibodies.
Primary culture and establishment of cell lines

Reporter gene? Recombinant protein expression? Antigen expression? Fluorescent labeling of an intracellular organelle? We help you to make the right cell for the right application.
Our research group is specialized in the genetic manipulation of cells and helps biotech companies to establish or improve specialized cells for their specific processes or applications. Our expertise includes the identification, the isolation and/or the immortalization of primary cells. Primary cells or already established clones can also be further manipulated to modify their characteristics, improve their performances or adapt them for new applications. Using this technology we can establish novel cell based assays, improve recombinant protein expression or develop cells with novel functions.
Induced pluripotent stemcells (iPSCs)
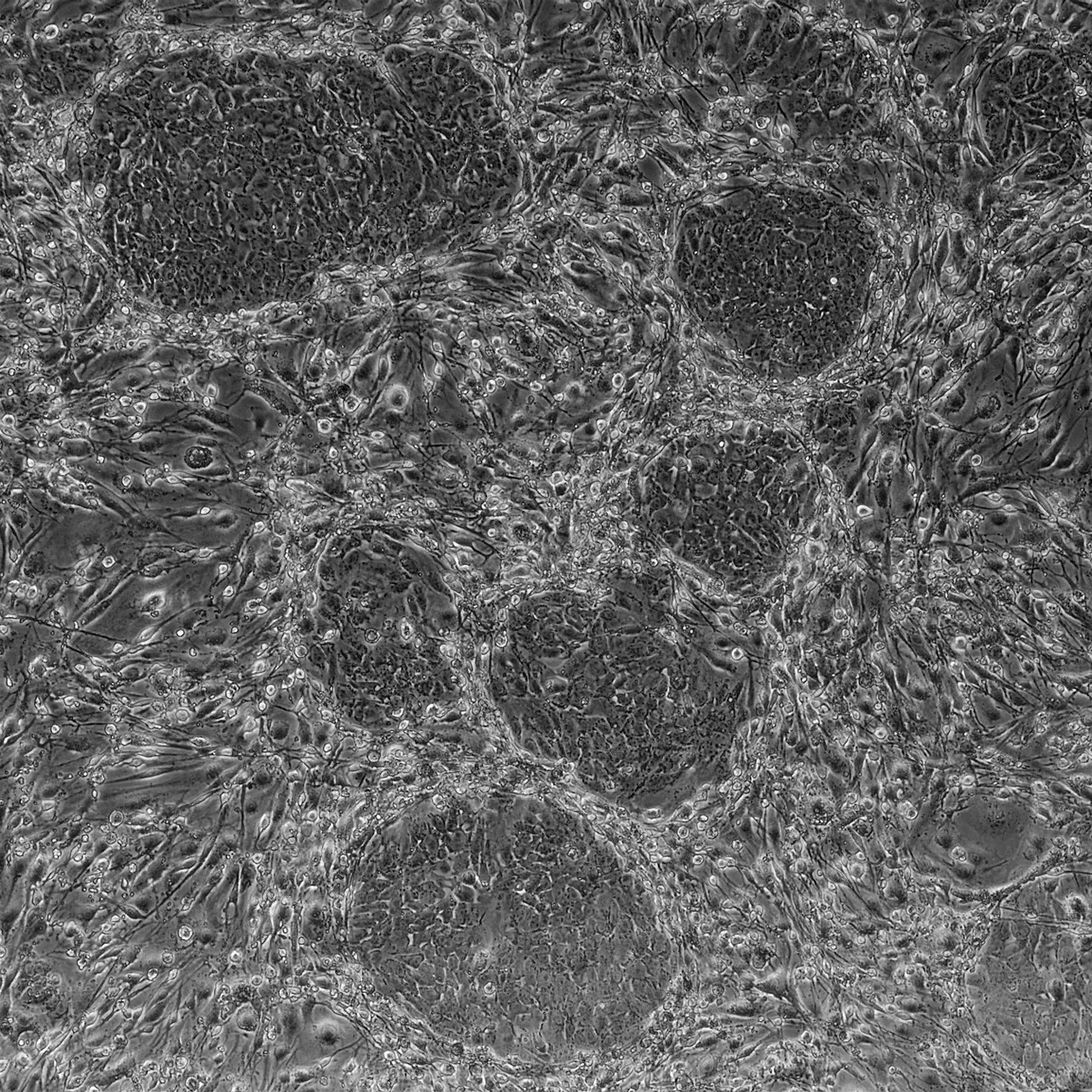
Pluripotent stem cells have an essential role in embryonic development as well as in tissue and organ homeostasis during the entire life of an organism. Understanding the biology of this outstanding kind of cell could provide the biotechnology industry with game-changing opportunities. Experts in regenerative medicine currently investigate possibilities to develop new organs or parts thereof using stem cells. On the other hand the cosmetic industry is strongly interested in the rejuvenation and regeneration potential for skin that this type of cells could provide. However work with stem cells is impaired by their natural scarcity as well as the technical and ethical difficulties to collect and cultivate them.
These obstacles were resolved in 2007 when Takahashi and colleagues showed that simple skin cells could be artificially induced into pluripotent stem cells. The pluripotency of those induced pluripotent stem cells (iPSCs) is best demonstrated by their ability, like a natural embryonic stem cell, to form viable mouse chimeras.
We established the technology to generate iPSCs at the ZHAW in order to bring the full potential of pluripotent stem cells to the biotechnology industry. The group of cell physiology and cellular engineering can therefore help its partners to develop applications based on stem cell biology such as:
- Test systems based on stem cells
- A steady supply of specialized cell types through targeted differentiation of iPSCs
- The identification and/or preparation of active ingredients from iPSCs
Recombinant protein expression and generation of respective cell lines
Nowadays proteins are produced recombinantly from suitable organisms such as E.coli, yeast cells, insect cells or mammalian cells.
In the group Cell physiology and cellular engineering we generate different mammalian cell lines that have the gene of the desired protein stably integrated into the genome and these cells secrete the proteins into the media. When the protein is a simple membrane protein or an intracellular protein, the protein sequence is slightly modified so that it will be secreted in the culture media for an easier purification scheme. The DNA sequence of the gene of interest can also be optimized for the codon usage to increase the yield of protein expression.
We also test the protein expression in small volumes and under different cultivation conditions from well plates up to spinner flasks. For each cell line the best growth media is used and the cells are incubated under optimal conditions.
Using biochemical methods, the protein is purified and tested for its quality and quantity.
Molecular Mechanisms of Cell Physiology for Biotechnical applications
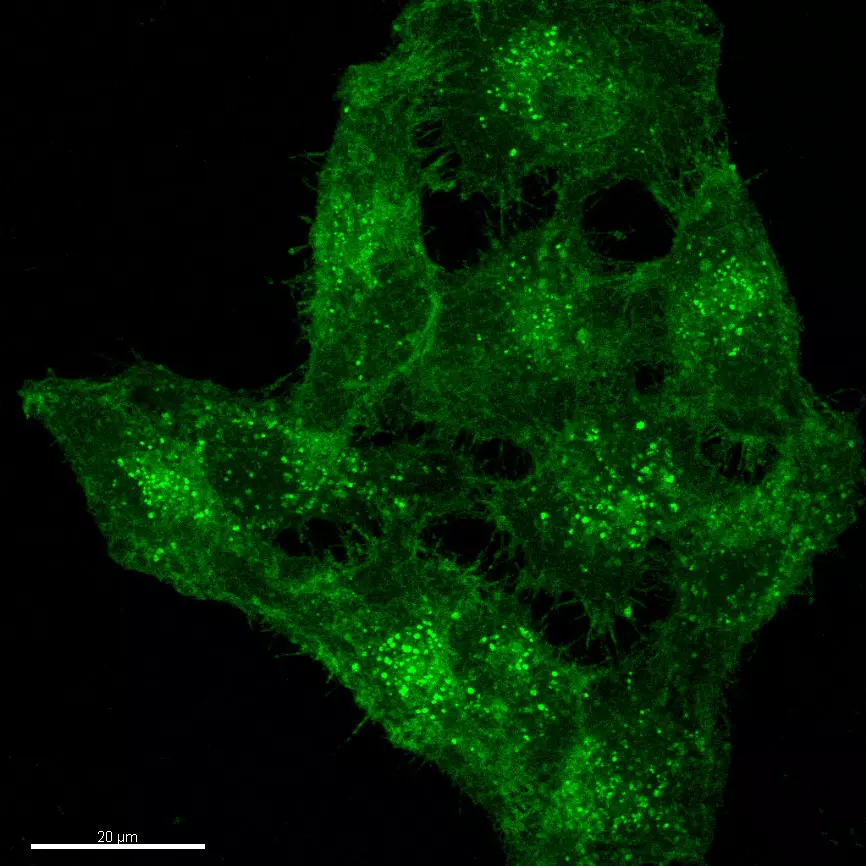
The molecular mechanisms of the cell physiology are often the basis for the understanding of essential processes in industrial biotechnology. Therefore the knowledge of the functional interactions within the cells and between the cells are the basis for the optimization of biotechnological processes or for the manufacture of products. Examples include changing an intracellular or membrane protein in a way that it is secreted by the cells for easier purification or changing the intracellular localization of an enzyme to use its activity for example as a membrane protein at the plasma membrane of special cells or to prevent wrong glycosylation of proteins which might be dangerous (uptake of the protein by dendritic cells and macrophages and creating an immune response) if used as a drug in humans.
Subcellular Drug Delivery
Many drug targets are localized within a specific organelle inside the cell. However, most drug delivery strategies are based on a systemic availability of the drug within the entire body rather than the specific delivery to the intracellular compartment. Such a specific transport to an intracellular target would allow for a drastically decreased effective concentration of the drug which would reduce or eliminate many side effects due to off-target interactions. Therefore the pharmaceutical industry is highly interested in such subcellular drug delivery systems. Having worked for more than 20 years in intracellular protein targeting we are working now in close collaboration with the research group Pharmaceutical Technology of Prof. Dr. Vera Luginbühl with their vast experience in Drug Delivery to address the issues of Subcellular Drug Delivery. Initially we would like to develop a system for the delivery of drugs to the endoplasmic reticulum and use the HMG-CoA reductase as a drug target for statins.
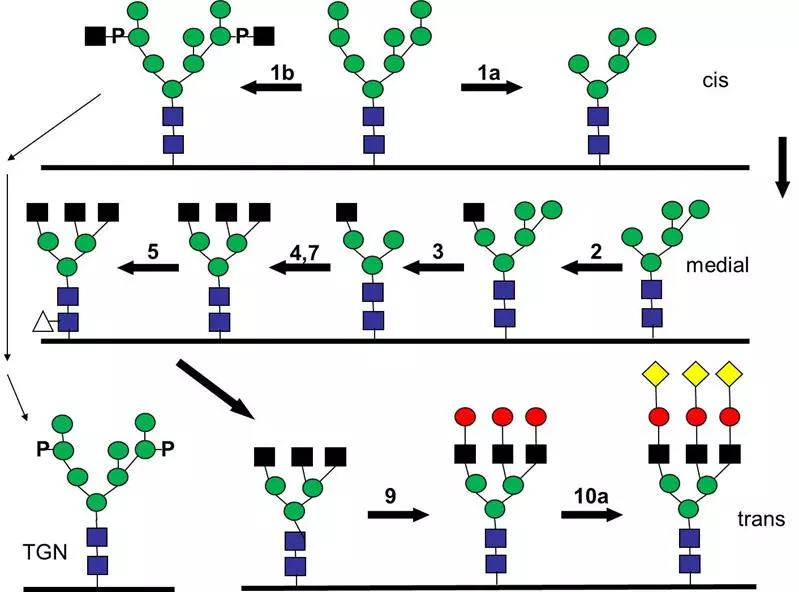
Glycobiology and post translational modifications
For projects in the area of glycobiology the intracellular transport and the correct localization of glycosyl transferases is determined as well as their enzymatic activity. The precise knowledge of the pathways allows a manipulation of the final localization of the glycosyl transferases for biotechnological use such as a secretion of the enzymes for easier purification. We also analyze the effect of differences in glycosylation of biosimilars (drugs, follow-on biologic, mostly a protein). The glycosylation of a protein as a hallmark of its quality, can vary during the production of recombinant proteins from producer to producer but also from batch to batch. This can lead to differences in the activity but also inherit the potential danger that the immune system of a patient will produce antibodies against those proteins used as drugs. In the best case such an immune reaction would render the drugs useless but unfortunately there is the danger that it will start an auto immune reaction.
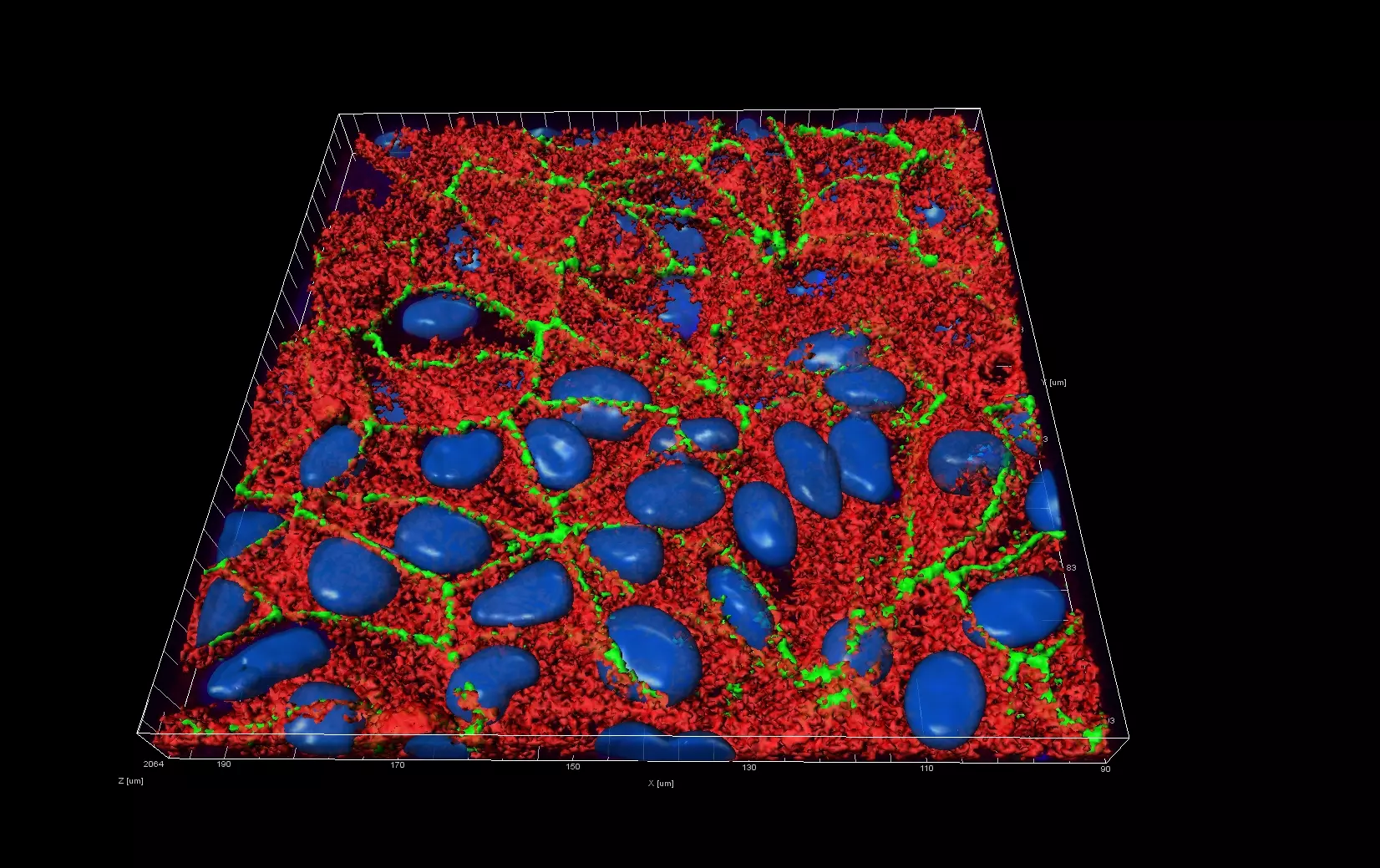
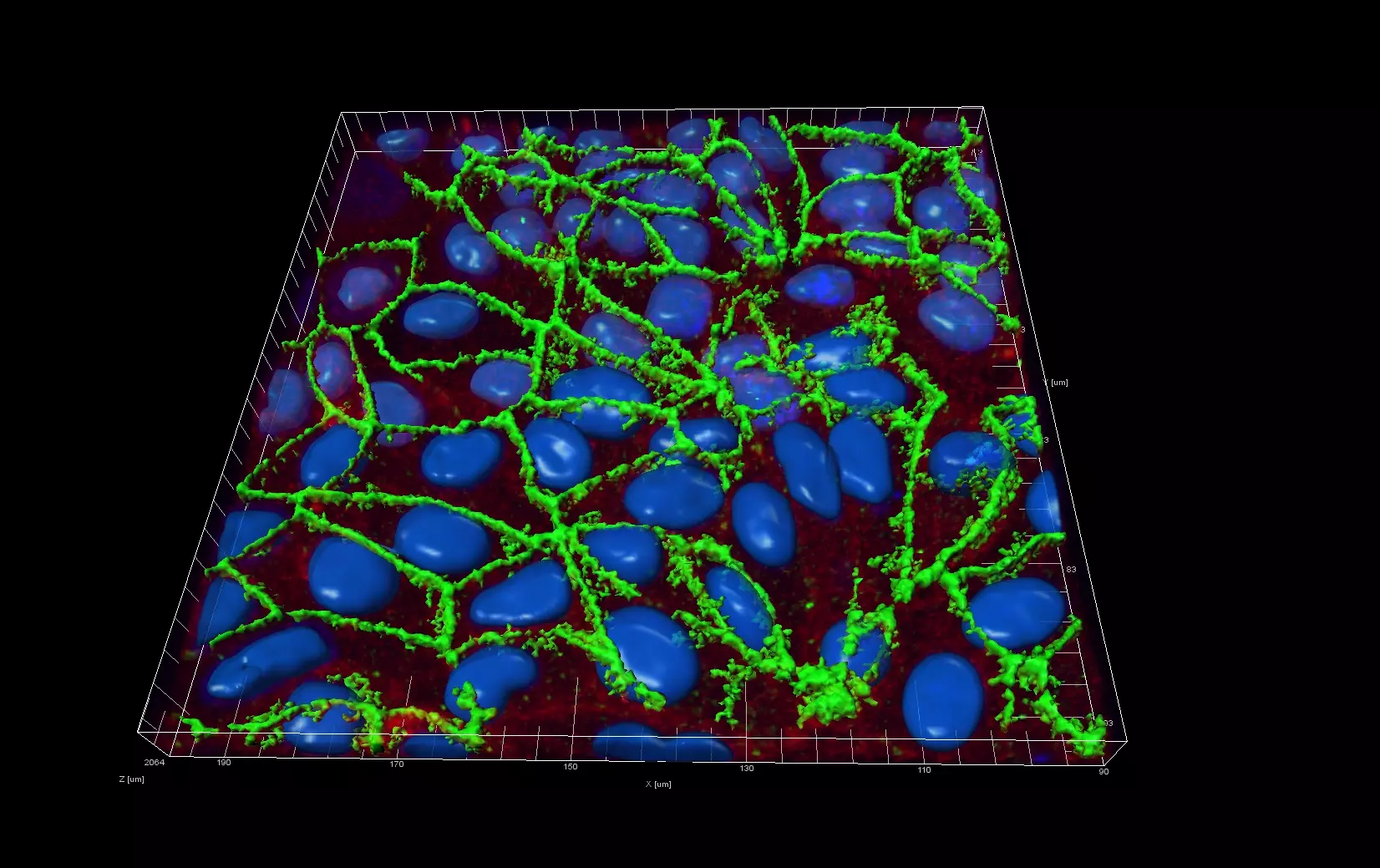
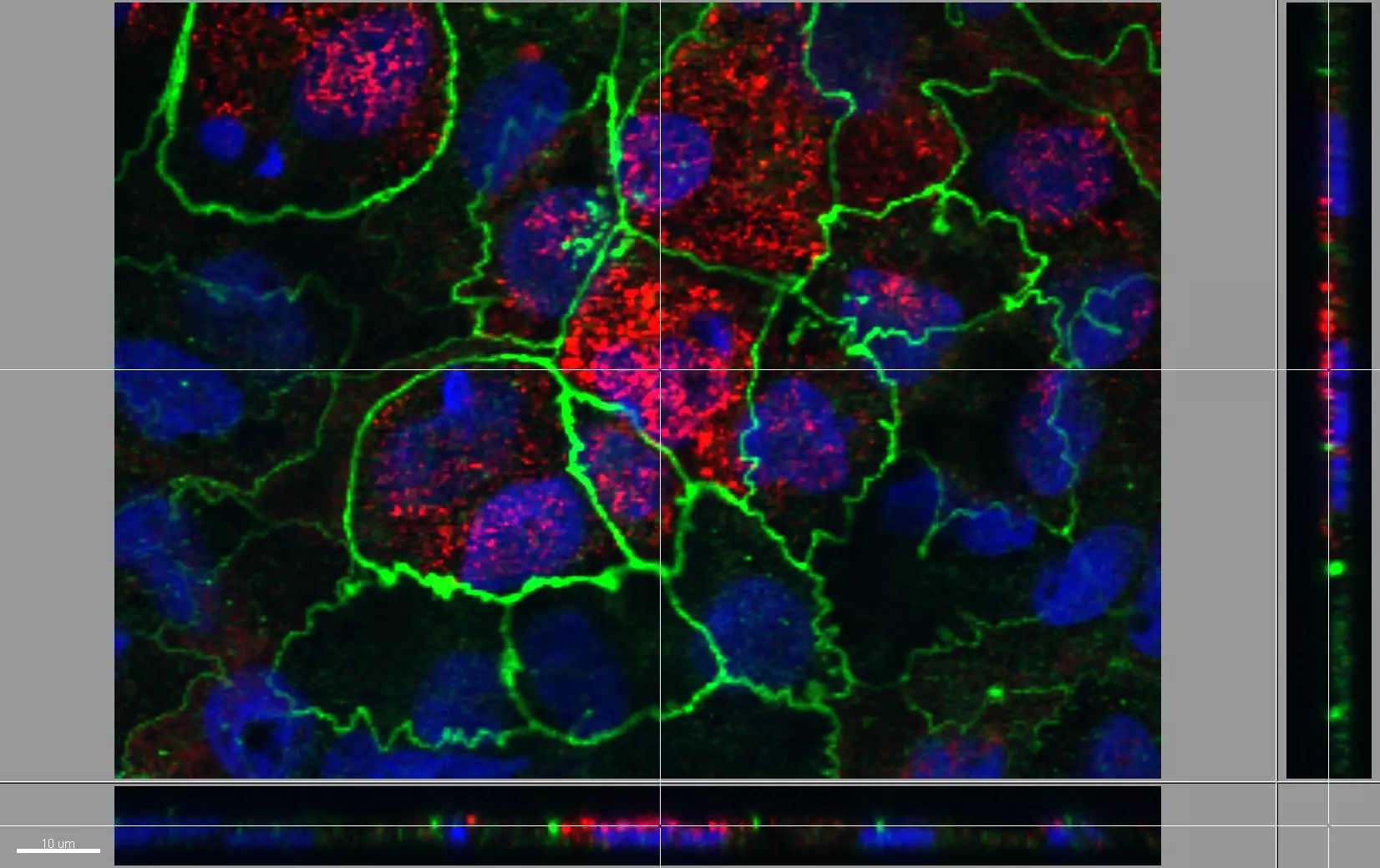
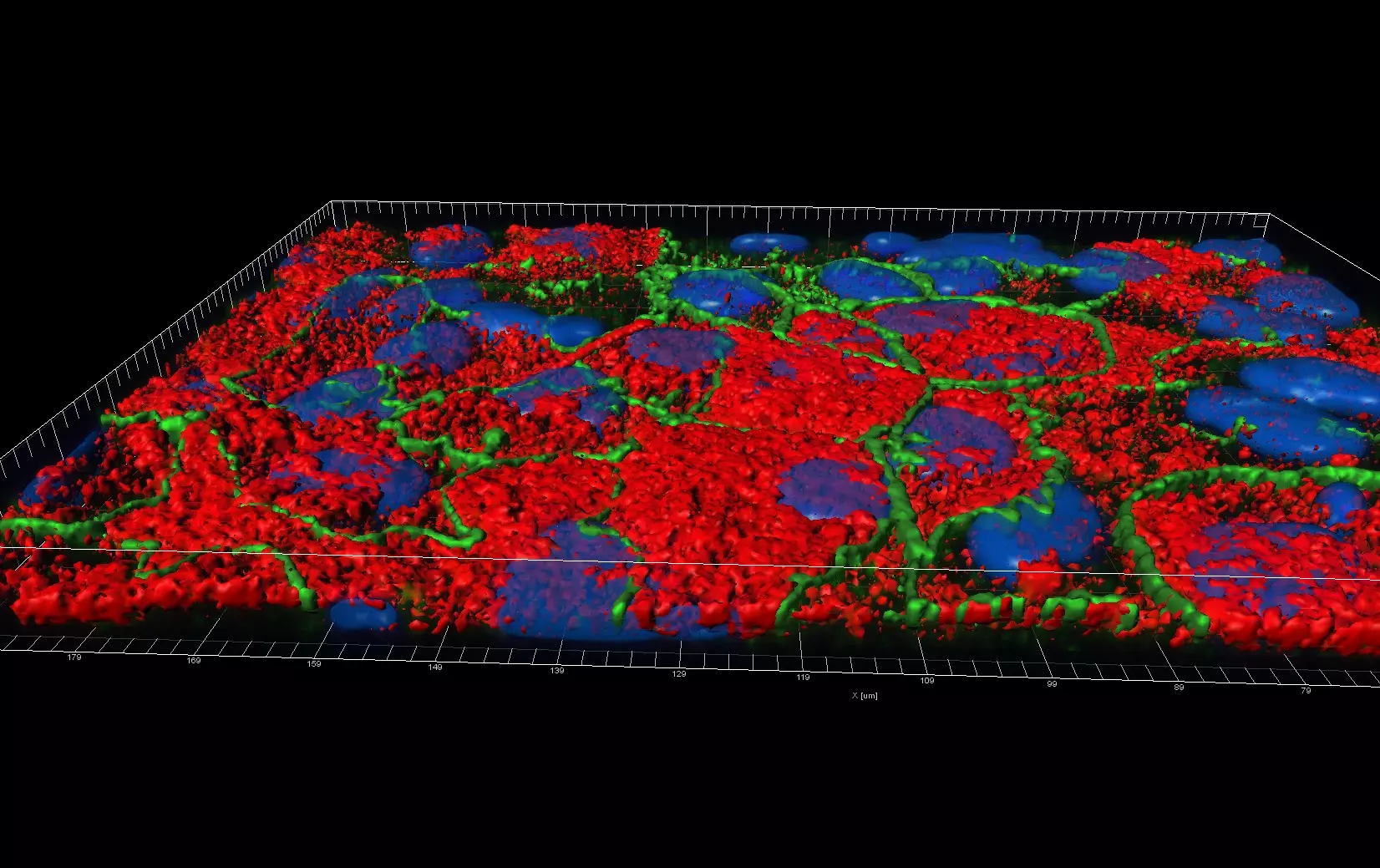
Live Cell Imaging

Unlike epifluorescence microscopy which uses light emitted by an halogen lamp, confocal laser microscopy uses a laser beam. The laser beam allows the focusing on a very thin layer of the sample, illuminating point by point in that layer. This avoids light to illuminate neighbouring areas of the sample.
The ZHAW possesses a confocal microscope FV1000 from Olympus equipped with a live imaging chamber.
Isolation and enrichment of specific cell types (FACS)
The laboratory of cell physiology and cell engineering manages a 4-lasers FACS (fluorescent activated cell sorter) that enables the precise analysis of physical or chemical characteristics of heterogeneous cell mixtures. Using the FACS we can identify rare cell types in a larger cell population (e.g. mesenchymal stem cells from adipose tissues), immuno-genotype cells (e.g. peripheral white blood cells), monitor cell differentiation (e.g. differentiation of macrophages to osteoclasts) or detect subtle changes in cellular physiology. Our flow cytometer is also equipped with an electrostatic sorting module that enable the enrichment of a specific cell type from a complex cell mixture or the sorting of a single cells into 96 well plates to generate a new clonal population.